Abstract
Introduction: The SAAWP and PDWP of the EBMT performed a retrospective registry study on the largest cohort of 813 children (< 18 years) with Fanconi anaemia (FA) undergoing first haematopoietic cell transplantation (HCT) in 137 centers between 2010 and 2018.
Methods: The primary endpoints were overall survival (OS), event-free survival (EFS; survival without graft failure, relapse and post-transplant malignancy) and GvHD-relapse free survival (GRFS; survival without grade III-IV acute GvHD (aGvHD), extensive chronic GvHD (cGVHD), graft failure, relapse and post-transplant malignancy). Secondary endpoints were cumulative incidence (CI) of grade II-IV aGvHD, cGvHD and graft failure. Subgroup differences in OS, EFS and GRFS were evaluated by log-rank test. Competing risks methods were used for the cumulative incidence of acute and chronic GVHD, with competing events being represented by death, graft failure, relapse and second transplant. Subgroup differences in cGvHD and aGvHD were evaluated by Gray's test. All estimates are reported with 95% confidence intervals.
Results: Indications of transplant were bone marrow failure (BMF, n=778, 96%) and AML/MDS (n =35, 4%). Median age at transplant was 8.8 years (IQR, 6.5-11.5) and median follow-up was 3.7 years (3.4-4.0). Median interval between diagnosis and HCT was 2.1 years (IQR 0.8-4.5). Donors were MSD (n=342, 42%), MRD (n=99, 12%), MMFD (n=23, 3%), haploidentical donor (HID, n =66, 8%), MUD (n=162, 20%), MMUD (n= 121, 15%). Stem cell source was bone marrow (n=526, 65%), peripheral blood (n=226, 28%) or cord blood (CB; n=51, 6%) or BM+CB (n=9, 1%). Conditioning regimen was FluCy (n=91; 11%), FluCy /others (n=585, n=72%) and others (n=137, 17%). Ninety-six (12%) received TBI. Serotherapy was ATG (n=623, 77%), alemtuzumab (n=86, n=11%) and none (n=102, 13%). Eighty-five (11%) received T-cell depleted graft.
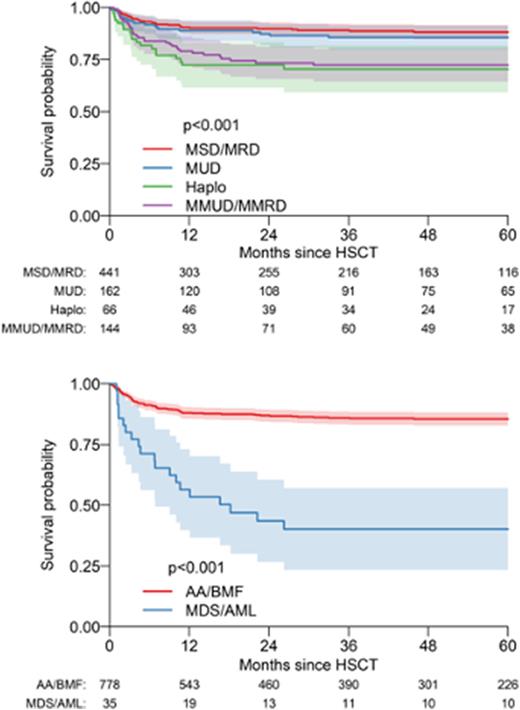
The 5-year OS, EFS and GRFS were 83% (95% CI, 80-86%), 78% (75-81%) and 70% (67-74%). On univariate analysis, indications (p<0.001), age at HCT (p=0.009), donor type (p<0.001), stem cell source (p=0.02), conditioning (p=0.02), TBI (p=0.004), were significantly associated with OS. On multivariate analysis, indication (compared to BMF, AML/MDS had significant inferior OS, HR 5.12, p<0.001; BMF, 85%, 83-89%; AML/MDS 40%, 23-57%), donor type (compared to MFD, patients transplanted with MMUD/MMFD and HID had significantly inferior OS (2.11, p<0.001 and 2.63, p=0.013 respectively); MFD: 88%, 85-91%; MUD: 86%, 80-91%; MMUD/MMFD: 72%, 64-80%; HID: 70%, 59-82%; p<0.001) and serotherapy (compared to ATG, patients transplanted with alemtuzumab had lower mortality (0.35 (0.15-0.8), p=0.01)) (ATG: 82%, 79-85%; alemtuzumab: 93%, 88-98%; none: 83%, 80-88%; p=0.04) had significant impact on OS.
In multivariate analysis, age at HCT, indications and donor type had significant impact on EFS and GRFS. In EFS, AML/MDS (HR=3.95, p<0.001) and older age (HR=1.76 per decade, p=0.015) were associated with worse outcomes. Event rate was significantly higher in HID and MMUD/MMFD (HR= 4.75 and 2.85 respectively, p<0.001). In GRFS, event rate was significantly higher in AML/MDS (HR=2.55, p<0.001) and all donor types compared to MFD (HR=2.05, 3.83 and 3.8 in MUD, HID and MMFD/MMUD respectively, p<0.001). Older age was associated with worse outcomes (HR=1.51 per decade, p=0.034). CI of grade II-IV aGvHD at day 100 and cGvHD at 1 year were 23% (20-26%) and 8% (6-10%). CI of secondary malignancies was 1% at one year and 3% at 5 years.
Conclusion: This study demonstrates that MFD and MUD had comparable survival in paediatric FA; early HCT using MUD should be offered in the presence of peripheral cytopenia, as older age at HCT and development of MDS/AML are associated with inferior survival.
Disclosures
Dalle:Teva: Current equity holder in private company; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria; Vertex: Honoraria; Sanofi: Honoraria; Medac: Honoraria; Orchard: Honoraria. Locatelli:GILEAD: Speakers Bureau; AMGEN: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NOVARTIS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; NEOVII: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MILTENYI: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MEDAC: Speakers Bureau; SANOFI: Membership on an entity's Board of Directors or advisory committees; SOBI: Speakers Bureau; BLUEBIRD BIO: Speakers Bureau; TAKEDA: Speakers Bureau; JAZZ PHARMACEUTICALS: Speakers Bureau; PFIZER: Membership on an entity's Board of Directors or advisory committees. Dufour:Biocryst: Consultancy; Pfizer: Consultancy; Gilhead: Consultancy; Novartis: Consultancy. Risitano:Sobi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Amyndas: Consultancy; Alexion: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Ra Pharma: Research Funding; Pfizer: Honoraria, Speakers Bureau; Alnylam: Research Funding; Apellis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Samsung: Membership on an entity's Board of Directors or advisory committees; Achillion: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.